Professor
Condensed Matter Physics
Department of Physics and Physical Oceanography
Memorial University of Newfoundland
 |
M. R. Morrow Professor Condensed Matter Physics Department of Physics and Physical Oceanography Memorial University of Newfoundland |
 |
|
Home Research Group Members and Recent Theses Publications Teaching Research in the Department Biophysics in Canada |
Lipids dispersed in water form bilayers which are useful models for biological membranes and for materials with internal conformational freedom. We study how bilayer properties like phase behavior, dynamics, and bilayer organization respond to interactions with membrane-associated proteins or changes in bilayer composition or pressure. For bilayers with deuterated components, wideline deuterium NMR observations reflect the extent to which the orientation-dependent quadrupole interaction is modulated by molecular motions. The bilayer state can then be characterized by the amplitudes and timescales for motions ranging from reorientation of molecular segments to bilayer collective motions. Phenomena studied may be relevant to biological function or to the material properties of membrane-mimetic soft materials. Insights or questions arising from a biological or material focus can motivate or inform the other aspect. Specific topics of interest include: Interactions relevant to organization and transformation in lung surfactant material
Phase transitions, dynamics, and reorganization in bicellar lipid mixturesInterest in mixtures of long and short chain lipids (bicellar mixtures) first arose because of their potential to form magnetically-orentiable phases that facilitate NMR studies of membrane proteins. The short chain lipids tend to separate into highly curved regions at the edge of disks, worm-like micelles, or pores. We are interested in such materials as models of lipid phases with highly curved edges and transitions that involve substantial reorganization and changes in aggregation. Insights into transitions that change the aggregation state of lipidic particles may be relevant to liposomal assembly and are being pursued further. We have used multiple pulse NMR observations to show that the slow motions in the high temperature lamellar phase of bicellar materials are significantly different from those in multilamellar dispersions of bilayers with more homogeneous chain-length distributions.  Lipid assembly responses to high pressureStudies of how soft materials respond to pressure, an important thermodynamic variable, have been limited by technical considerations. This is one of a few labs, worldwide, with the capacity for solid-state NMR studies of soft materials at high pressure. Lipid bilayers are anisotropic. Their responses to hydrostatic pressure reflect competition between lateral compression (increased chain order/extension) and longitudinal compression (bilayer thinning). This competition can result in transitions, at high pressure, to phases, such as the interdigitated gel phase in which lipid chains extend past the bilayer mid-plane, that are not seen at ambient pressure. This can provide new insights into bilayer material properties. For example, DPPC and DPPG, which differ primarily in headgroup charge, behave similarly at ambient pressure but not at high pressure. We have found that DPPG (negative headgroup) interdigitates at lower pressure than DPPC (zwitterionic) suggesting that repulsion shifts the balance between lateral and longitudinal compression. Variable pressure may facilitate the study of bilayer reorganization processes having interdigitated phases as possible intermediate states. We have also found evidence that increased chain unsaturation reduces sensitivity of bilayer order to pressure and extends earlier work by this group on ambient-pressure phase behaviour of unsaturated lipid bilayers. 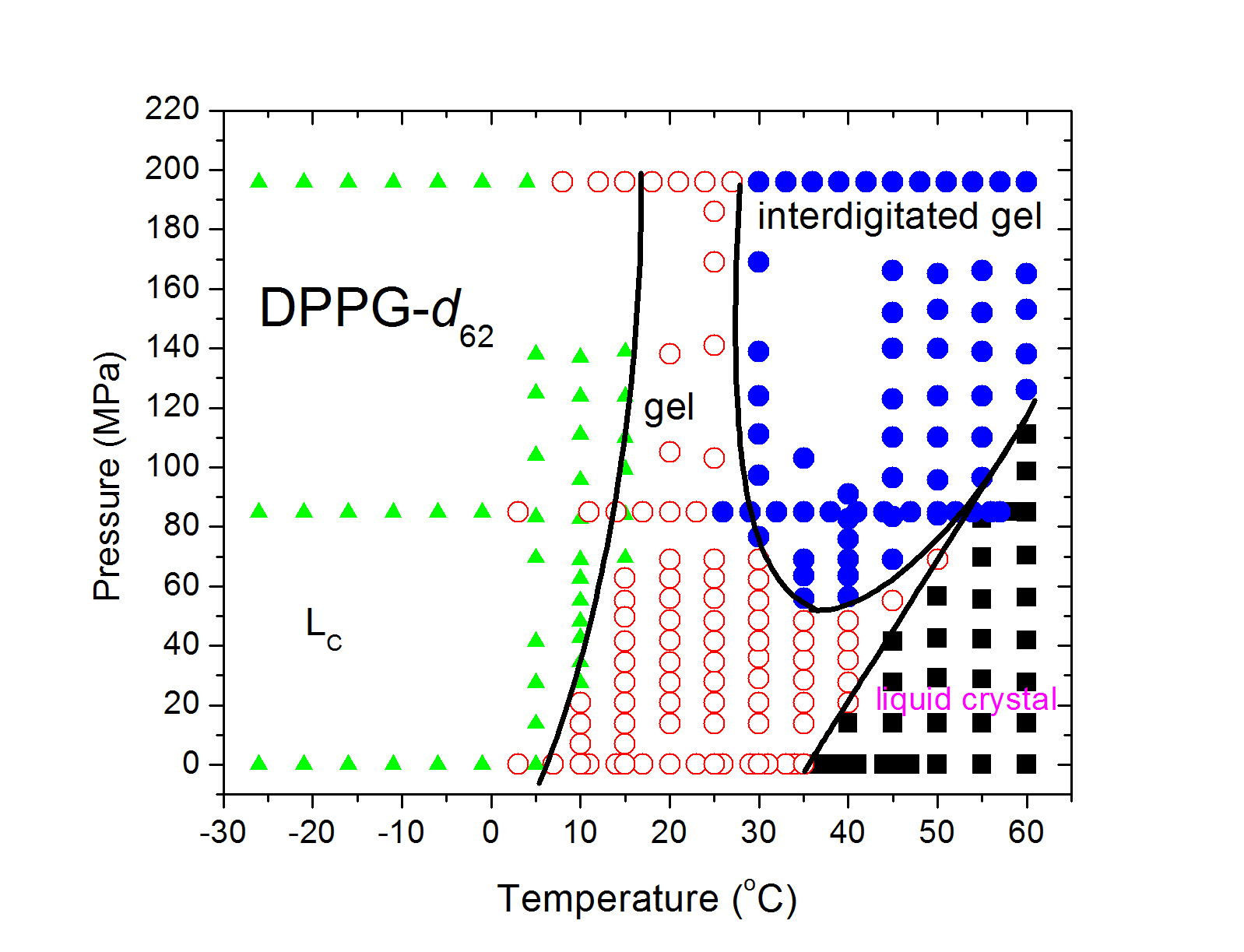 Orientation and dynamics of membrane-associated polypeptides
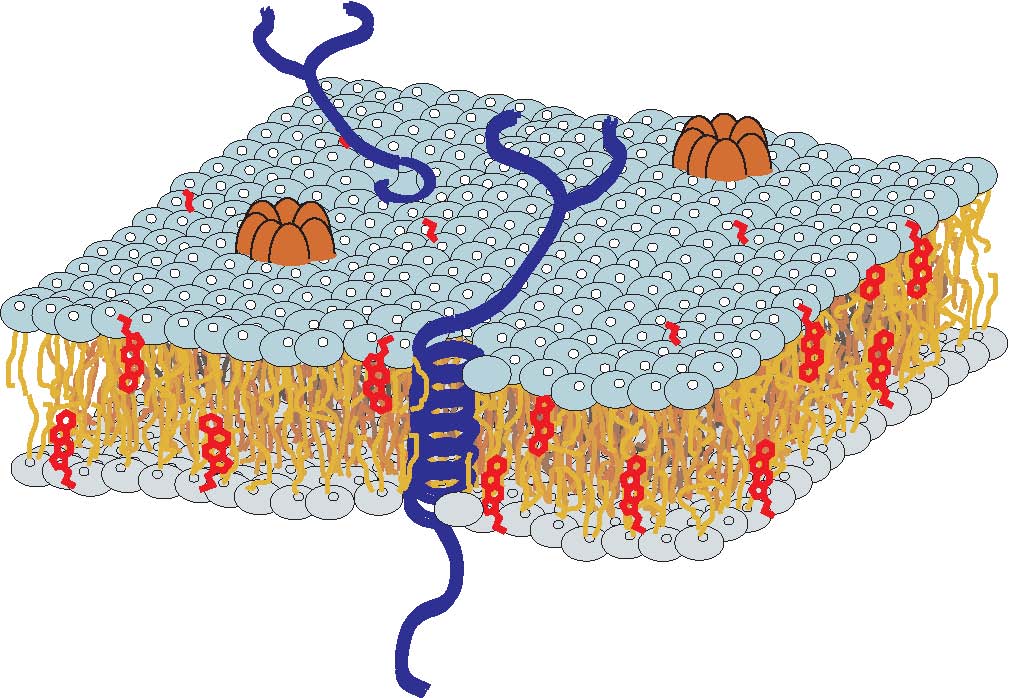
Perturbation of bacterial membranes by antimicrobial peptides
FacilitiesIn the past, our primary tools were two wideline NMR spectrometers built by M. Morrow and students. One based on a 3.5 T widebore superconducting magnet was used primarily for variable pressure studies of samples containing deuterium-labeled lipids. These spectrometers have been decommissioned. Students now have access to a 14.1 T Bruker Avance II NMR spectrometer. |
|||||||